Dermatology Question of the Week: Deductive Dermpath

A 10-year-old patient presents to your office with the following red papule on their nose. A shave biopsy shows a proliferation of melanocytes.
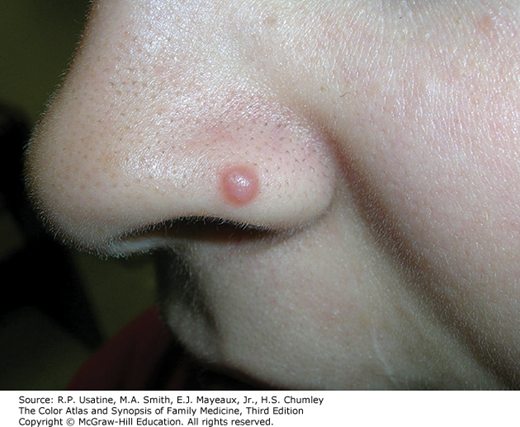
Which of the following features would be reassuring for a Spitz nevus rather than melanoma? (hint: more than one may be correct)
A. Loss of p16
B. Sharply circumscribed
C. High mitotic rate
D. Vertically oriented nests with clefting
E. Presence of Kamino bodies
Rationale: Spitz nevi were characterized by Dr. Sophie Spitz in 1948. Spitz nevi exist upon a spectrum of melanocytic neoplasms which include atypical Spitz tumor and Spitzoid melanoma. Identification is important as Spitz nevi typically have very, very low risk of progression by comparison to melanoma. Several features to distinguish the features have been described. Clinically, Spitz nevi present as a dome-shaped papule less than 1cm in size that may be darkly pigmented or have more of a reddish/skin-colored appearance.
Correct answers: B, D, E
Spitz nevi are characterized by multiple features including their sharply circumscribed nature, vertically oriented nests with clefting, and eosinophilic hyaline granules known as Kamino bodies. Additional features and comparisons to atypical Spitz tumor and Spitzoid melanoma are shown in the table below.
Table 27-19 Comparison of Spitz Nevus, Atypical Spitz Tumor, and Spitz Melanoma
| SPITZ NEVUS | ATYPICAL SPITZ TUMOR | SPITZ MELANOMA | |
|---|---|---|---|
| Clinical features |
Mean and median age 21 years (range 2 to 69 years) Extremities most common Pink or reddish plaque, papule, or nodule |
Any age, younger patients <40 years Extremities, trunk Plaque or nodule Color variegation |
Any age, often >40 years Extremities, trunk Asymmetrical Enlarged plaque or nodule Color variegation Changing lesion |
| Histopathology |
Size <5 to 6 mm Symmetrical Well circumscribed Epidermal hyperplasia Vertically oriented nests with clefting Central focal pagetoid spread if any Often wedge shaped Maturation of dermal component Few or no dermal mitoses (0 to 2 per mm2) |
Often >5 to 10 mm Symmetrical or asymmetrical Well or poorly circumscribed Ulceration possible Irregular nesting Greater cellularity Greater pagetoid spread Deeper dermal extension Maturation may be partial or absent Dermal mitoses 2 to 6 per mm2 Deep mitoses Possible necrosis |
>5mm, often >10 mm Often asymmetrical Often poorly circumscribed Ulceration Irregular and confluent nesting Pagetoid spread may be extensive Ulceration Effacement of epidermis Lack of maturation Dermal mitoses often >6 per mm2 Mitoses deep/marginal, or atypical Necrosis |
| Cytology |
Enlarged epithelioid/spindle cells Little or no nuclear pleomorphism Absence of high-grade cytological atypia |
Enlarged epithelioid/spindle cells Nuclear enlargement, pleomorphism, hyperchromasia |
Enlarged epithelioid/spindle cells High-grade cytological atypia |
| Immunohistochemistry |
HMB45 and Ki67 expression diminished with depth in dermal component Ki67 index low (<5%) |
HMB45 and Ki67 expression diminished or variable with depth Ki67 index low or intermediate (5% to 15%) |
HMB45 and Ki67 deep expression often Elevated Ki67 proliferative index (>20 %) p16 expression may be diminished or absent |
| Molecular |
Array CGH: isolated gains of 7p, 11q, tetraploidy HRAS-activating mutations Kinase fusions |
Array CGH: often 1 or multiple chromosomal abnormalities Kinase fusions PTEN mutations Heterozygous or homozygous loss of 9p21 may occur |
Array CGH: multiple chromosomal abnormalities Kinase fusions BRAF, NRAS mutations rare HRAS mutations rare PTEN mutations Homozygous loss of 9p21 TERT promoter mutations |
| Prognosis | Very low (almost no) risk of progression |
Low risk of progression Almost always indolent Clinical recurrences occur |
Regional clinical lymph node metastases occur Rare distant metastases and death |
Abbreviation: array CGH = comparative genomic hybridization.
Incorrect answers:
A. Loss of p16. Loss of p16 is a feature of melanoma whereas retention of p16 is a feature of Spitz nevus. p16 is located on chromosome 9 and is encoded by the CDKN2A gene. p16 functions as a tumor suppressor, hence the loss of p16 in melanoma leads to lack of tumor suppression which is not a finding typically seen in Spitz nevi.
C. High mitotic rate. Spitz nevi typically have a very low mitotic rate.
Additional reading at Barnhill's Dermatopathology Chapter 27: Tumors of Melanocytes

Create a Free MyAccess Profile
AccessMedicine Network is the place to keep up on new releases for the Access products, get short form didactic content, read up on practice impacting highlights, and watch video featuring authors of your favorite books in medicine. Create a MyAccess profile and follow our contributors to stay informed via email updates.